Turning to nutrition to help address complications
Since 2012, the American Academy of Pediatrics has recommended human milk as the primary source of nutrition for premature babies, with appropriate fortification for those born weighing < 1,500 g.1 Fortification with human milk-based fortifiers is the only way to ensure these medically fragile infants receive an exclusive human milk diet (EHMD).
In 2025, the US Food and Drug Administration approved the first human milk-based fortifier for use in term infants with Gastroschisis following surgical repair.
Learn more
Prolacta products used as a part of that EHMD,2 as compared to cow’s milk-based fortifiers, have been shown in clinical studies to benefit critically ill, premature infants in the NICU weighing ≤1250 g at birth in many ways including to:
Getting the most out of an EHMD
Leading the human milk industry in quality and safety
First and only pharmaceutical-grade manufacturing facilities for the testing and processing of human milk
Exceed food-product industry requirements by following stringent quality and safety standards based on those for the human plasma and blood industry in the U.S.
FDA-regulated lab processes
Our human milk laboratory and manufacturing processes are regulated by the U.S. Food and Drug Administration (FDA).
All Prolacta nutritional products are pasteurised to ensure the highest quality and safety, using time and temperature profiles defined by the FDA in its Pasteurized Milk Ordinance (PMO) to destroy pathogenic viruses and bacteria.
While there are other pasteurisation and sterilisation processes used in the human milk banking industry, only products manufactured with our pasteurisation process are clinically shown to improve health, and reduce complications, when used as part of an EHMD in the hospital. There is no clinical data showing similar results using products processed by any other method.
Benefits of HMOs
Prolacta’s 100% human milk-based nutritional products contain a wide spectrum of human milk oligosaccharides (HMOs).13 The immunity, prebiotic and gut maturation benefits that HMOs promote may have a role in the health outcomes attributed to an EHMD, including Prolacta’s products.14
To learn more

Improving health outcomes
For more than two decades, we have been advancing the science of human milk to improve the health of premature, critically ill infants worldwide.
- The clinical benefits of our products have been evaluated in 30+ clinical trial studies
- Globally, more than 100,000 premature infants have benefited from our proven human milk-based nutritional products15
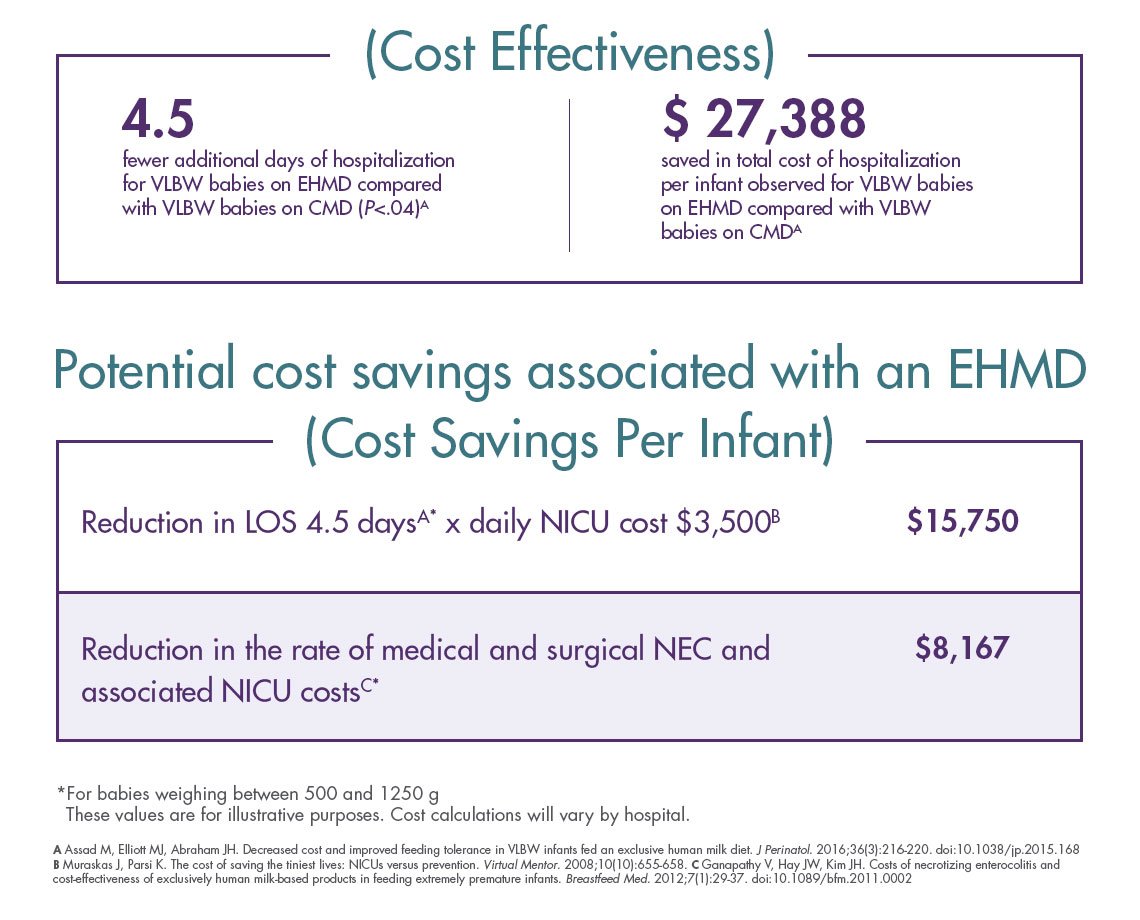
A cost-effective solution
An EHMD can increase survival5 and reduce the overall cost of care for premature infants weighing ≤1250 g at birth.12 With Prolacta’s products, as part of an EHMD (Prolacta’s EHMD),† you can decrease costly complications —such as necrotising enterocolitis — associated with cow milk–based products.6 When used as part of an EHMD, our nutritional products are clinically proven to increase survival and reduce the overall cost of care compared to cow milk–based fortifiers or preterm formula in extremely premature infants.
*Estimated number of premature infants fed Prolacta’s products from January 2007 to August 2023; data on file.
