Unlock the power of human milk
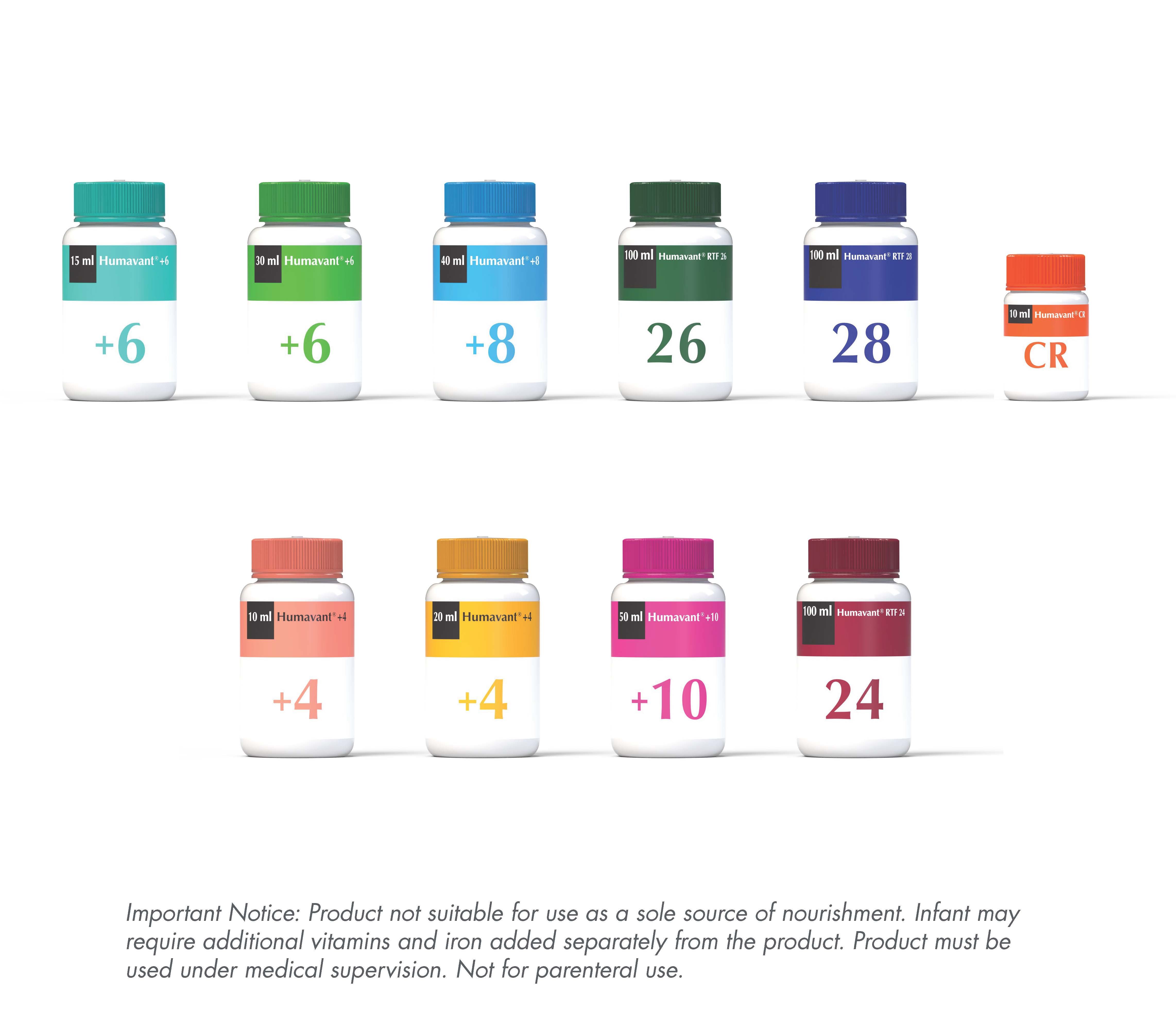
Optimal nutrition products
Humavant ® +6 HMF (15 ml) Lacto-engineered Human Milk-based Fortifier (Human, Pasteruised)
Humavant® +6 HMF (30 ml) Lacto-engineered Human Milk-based Fortifier (Human, Pasteruised)
Humavant® +8 HMF (40 ml) Lacto-engineered Human Milk-based Fortifier (Human, Pasteruised)
Humavant® RTF 26 (100 ml) Lacto-engineered Human Milk-based Fortifier, Premature Infant Formula
Humavant® RTF 28 (100 ml) Lacto-engineered Human Milk-based Fortifier, Premature Infant Formula
Humavant® CR (10 ml) Lacto-engineered Human Milk-based Fortifier (Human, Pasteruised)
Additional products for feeding flexibility
Humavant ® +4 HMF (10 ml) Lacto-engineered Human Milk-based Fortifier (Human, Pasteruised)
Humavant ® +4 HMF (20 ml) Lacto-engineered Human Milk-based Fortifier (Human, Pasteruised)
Humavant ® +10 HMF (50 ml) Lacto-engineered Human Milk-based Fortifier (Human, Pasteruised)
Humavant® RTF 24 (100 ml) Lacto-engineered Human Milk-based Fortifier, Premature Infant Formula
