Fact Sheet
Low birth weight premature infants face significant risks due to late, inadequate, and inappropriate nutrition. Fortunately, by focusing on nutrition, neonatal intensive care units are seeing promising outcomes. According to multiple studies, an exclusive human milk diet (EHMD) can significantly reduce bronchopulmonary dysplasia (BPD), one of the most serious complications of prematurity. When premature infants are fed human milk–based fortifiers from Prolacta Bioscience®, the incidence of BPD is 9.7% lower, per a weighted average, than that of those who receive cow milk–based fortifiers.1,2,3
The lungs are among the last organs to fully develop in the womb, leaving many premature infants at high risk of developing BPD. That’s why many hospitals across the country are working to curb the incidence of BPD as part of their quality improvement initiatives.
Infants with a BPD diagnosis face a number of complications, including:
BPD is a risk factor for persistent and severe respiratory morbidity at 1 year of age, including hospitalizations, exposure to systemic steroids or pulmonary vasodilators, home oxygen after 3 months or mechanical ventilation, or symptoms despite inhaled corticosteroids.4
During the first year of life, very low birth weight (VLBW) infants with BPD have longer hospital stays and more readmissions—leading to a 54% increase in hospitalization costs compared with VLBW infants without BPD.5
Premature infants with severe BPD may be at an elevated risk for cerebral palsy,6 delays in cognition and education,7,8,9,10 and attention deficit disorder.11
With the use of Prolacta’s products as part of an EHMD, hospitals can improve the quality of their patients’ lives for years to come. Beyond the short-term benefits of lowering BPD incidence1,2,3 and helping to address the nutritional risks associated with feeding intolerance,3 Prolacta products can help improve children’s prospects for normal cognitive development and educational success, benefiting families, communities, and health systems.
In three recent studies, the use of Prolacta’s fortifiers as part of an EHMD led to statistically significant decreases in BPD (weighted average of 9.7%) among premature infants compared to those fed cow milk-based fortifiers.1,2,3 Another study revealed that time is of the essence: Early fortification with Prolacta’s fortifiers as part of an EHMD led to a 15% reduction in the incidence of BPD, compared with late fortification.12
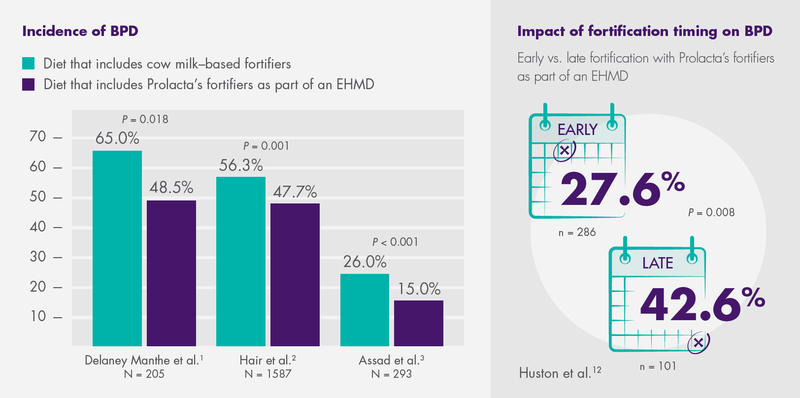
1 Delaney Manthe E, Perks PH, Swanson JR. Team-based implementation of an exclusive human milk diet. Adv Neonatal Care. 2019;19(6):460-467. doi:10.1097/ANC.0000000000000676
2 Hair AB, Peluso AM, Hawthorne KM, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed Med. 2016;11(2):70-74. doi:10.1089/bfm.2015.0134. Published correction appears in Breastfeed Med. 2017;12(10):663. doi:10.1089/bfm.2015.0134.correx
3 Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36(3):216-220. doi:10.1038/jp.2015.168
4 Keller RL, Feng R, DeMauro SB, et al. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J Pediatr. 2017;187:89-97.e3. doi:10.1016/j.jpeds.2017.04.026
5 Lapcharoensap W, Bennett MV, Xu X, Lee HC, Dukhovny D. Hospitalization costs associated with bronchopulmonary dysplasia in the first year of life. J Perinatol. 2020;40(1):130-137. doi:10.1038/s41372-019-0548-x
6 Van Marter LJ, Kuban KC, Allred E, et al. Does bronchopulmonary dysplasia contribute to the occurrence of cerebral palsy among infants born before 28 weeks of gestation? Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F20-F29. doi:10.1136/adc.2010.183012
7 Jeng SF, Hsu CH, Tsao PN, et al. Bronchopulmonary dysplasia predicts adverse developmental and clinical outcomes in very-low-birthweight infants. Dev Med Child Neurol. 2008;50(1):51-57. doi:10.1111/j.1469-8749.2007.02011.x
8 Natarajan G, Pappas A, Shankaran S, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88(7):509-515. doi:10.1016/j.earlhumdev.2011.12.013
9 Short EJ, Kirchner HL, Asaad GR, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med. 2007;161(11):1082-1087. doi:10.1001/archpedi.161.11.1082
10 Gray PH, O’Callaghan MJ, Rogers YM. Psychoeducational outcome at school age of preterm infants with bronchopulmonary dysplasia. J Paediatr Child Health. 2004;40(3):114-120. doi:10.1111/j.1440-1754.2004.00310.x
11 Astbury J, Orgill AA, Bajuk B, Yu VY. Neonatal and neurodevelopmental significance of behaviour in very low birthweight children. Early Hum Dev. 1985;11(2):113-121. doi:10.1016/0378-3782(85)90098-2
12 Huston R, Lee M, Rider E, et al. Early fortification of enteral feedings for infants <1250 grams birth weight receiving a human milk diet including human milk based fortifier. J Neonatal Perinatal Med. 2020;13(2):215-221.doi:10.3233/NPM-190300
13 Estimated number of premature infants fed Prolacta’s products from January 2007 to August 2020; data on file.
Established in 1999, we created the first and only neonatal nutritional fortifiers made from 100% human milk, rather than cow milk. Based in California with employees throughout the world, we’re a privately held life sciences company that’s touched the lives of more than 63,000 premature infants globally.13 We operate the first and only pharmaceutical-grade manufacturing facilities for testing and processing donor milk. We exceed food-product industry requirements by following stringent quality and safety standards based on those for the human blood and plasma industry.