Not all donor milk is equal: Impact of processing methods on bioactive compounds
Diane L Spatz, PhD, RN-BC, FAAN and Kelley Baumgartel, PhD, RN
Diane L Spatz is a Professor of Perinatal Nursing at the College of Nursing University of South Florida. Kelley Baumgartel is an Assistant Professor at the College of Nursing University of South Florida.
Background
The importance of human milk for both healthy and vulnerable infants in order to improve health and developmental outcomes in infancy, throughout childhood, and into adulthood has been well documented, yet breastfeeding rates remain suboptimal.1 Parents need to receive evidence-based care, assistance and education in order to both make an informed feeding choice and effectively initiate and maintain milk supply.2,3 The Spatz 10 step model for human milk and breastfeeding has been effectively implemented in hospitals both in the United States and globally with improved initiation rates and improved human milk rates at discharge and beyond.2,3,4,5,6 Unfortunately, the use of human milk and breastfeeding has not been universally prioritized and there remain persistent health disparities and barriers to achieve global breastfeeding recommendations for initiation, exclusivity and duration.1 Therefore, many infants are exposed to infant formula early in life. This is particularly concerning given the amount of research that exists on the benefits of human milk for vulnerable infants. See Table 1.
Without enough mother’s own milk, it is important to prioritize the use of Pasteurized Donor Human Milk (PDHM) as a bridge. In both the United States and worldwide more milk banks are being established. There has been an increase in both non-profit and for-profit milk banks. Despite the 2017 American Academy of Pediatrics (AAP) position statement focusing on the quality and safety of Pasteurized Donor Human Milk (PDHM) and more hospitals using PDHM, there remain persistent disparities in the use of PDHM.7,8

Types of Donor Milk
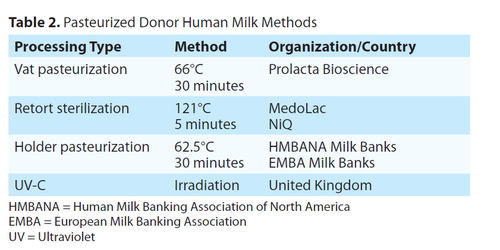
In addition to the increased interest and use in PDHM, there are several for profit companies that have opened and are aggressively marketing and selling retort sterilized milk. Unlike pasteurization, the purpose of retort processing is sterilization which destroys many protective human milk compounds. In contrast, the purpose of pasteurization is to kill harmful organisms. There are two types of pasteurization, vat and Holder. This article aims to help readers understand that not all “donor milk” is equal. The different ways in which milk is processed is not well understood by most clinicians, nor do they understand the impact of the different methods on the bioactive components of human milk. Table 2 details the various methods of processing.
Nurses in particular have voiced concerns regarding changes in practice when their hospital changed from PDHM to commercially sterile milk.9 In particular, nurses verbalized anger and frustration because they were not involved in the decision-making processes.9 Furthermore, most nurses believed the change was made to save money and this was viewed negatively.9 Nurses also voiced confidence in the use of PDHM but were uncertain about commercially sterile milk.9 Nurses strongly wanted evidence to support a practice change from the use of PDHM to commercially sterile milk but felt they had to go along with the change because there were barriers to obtaining the necessary evidence.9
Fortifiers for Human Milk
In addition to the expansion of PDHM, there has also been an increase in the use of human milk-based fortifiers for preterm or other critically ill infants. Human milk-based fortifiers are used primarily to improve growth. Traditional fortifiers are made from bovine products. Prolacta Bioscience is the single manufacturer of human milk-based fortifiers which have been in use in the United States since 2006. These human milk-based fortifiers are processed using the vat pasteurization process, therefore many of the bioactive components of human milk are maintained and exposure to bovine products is prevented.
Research reports that extremely premature infants who receive diets devoid of bovine products have lower rates of morbidity and mortality.10 Additionally, Hair & colleagues report improved growth for infants under 1250 grams for those who received an exclusive human milk diet (with human milk fortifiers) compared to a prior cohort of infants.11
Milk Processing & Bioactive Components of Human Milk
Human milk is a highly complex biological system with variable composition that is influenced by many factors. Vat pasteurization and retort sterilization methods have varying effects on bioactive human milk compounds. Overall, vat pasteurization of donor milk and human-milk based fortifiers results in the protection of many such bioactive compounds. For example, immunoglobulins provide passive immunity by way of human milk to the preterm infant who has an underdeveloped immune system. Secretory IgA helps to establish the intestinal microbiome,12,13,14 with robust antimicrobial,15 and anti-inflammatory components.14
IgM, also found in human milk, prevents the activation of the complement system.16 IgG is transferred to the infant circulation via human milk and acts directly on the bacteria found within the intestine. This immunoglobulin establishes bacterial colonization12 by way of opsonization, which prevents acute inflammatory responses.14 All immunoglobulins are vulnerable to decreased activity secondary to heat; however, different processing techniques result in varying immunoglobulin concentration. Vat pasteurization results in higher immunoglobulin amount and activity when compared with retort sterilization. Specifically, retort sterilization has a significantly negative impact on sIgA, resulting in 90% less sIgA and significantly decreased bioactivity.17 IgM is also impacted by heat, with levels higher in vat pasteurized milk when compared with retort sterilization. Similarly, milk IgG is higher after vat pasteurization when compared with retort sterilization.18
Lysozyme is an antimicrobial enzyme that degrades bacteria and eliminates stimuli that lead to acute inflammatory responses.14 Lysozyme works together with lactoferrin to reduce and eliminate pathogenic microbes. To do this, lactoferrin binds with iron, thereby eliminating it from the circulation for bacterial use.12 Lactoferrin also prevents biofilm formation in the gastrointestinal tract and induces macrophage activity.12 Lysosomal activity is absent following retort sterilization. Vat pasteurization results in higher milk levels of both lysozyme17 and lactoferrin.18
Additional bioactive protein concentration and activity levels are also greatly impacted by different processing methods. Alpha-lactalbumin binds and assists with antibiotic uptake, thereby inhibiting bacterial reproduction18 during times of gastrointestinal inflammation and potential pathology. Alpha-antitrypsin also prevents bacterial-induced pathology by limiting the ability of pathogens to enter the body,14 as well as limiting local inflammation. Osteopontin, is a highly bioactive milk compound that promotes optimal immune and gastrointestinal environments. Significant to preterm infants, osteopontin supports brain development.19 Working together with these proteins, casein, which represents more than half of milk protein content, contributes to the overall antimicrobial activity of human milk.18 Human milk levels of alpha-lactalbumin, alphaantitrypsin, osteopontin, and casein, are all higher when vat pasteurization is used, when compared with the retort sterilization method.
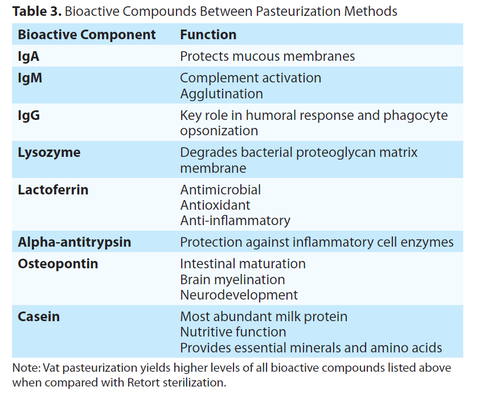
Human milk oligosaccharides (HMOs) are a critical bioactive component in human milk, as they greatly contribute to the microbial establishment of commensal bacteria. They often act as a decoy for pathogens,14 which inhibits their ability to adhere to the gastrointestinal wall.15 When compared with retort sterilization, vat pasteurization results in higher levels of HMOs. This is highly relevant to preterm infant health, as the milk microbiome is depleted with any other processing method which uses heat. The conservation of HMOs provides vulnerable infants with a robust and diverse gastrointestinal microbiome. HMOs are significantly higher in concentration following vat pasteurization versus retort sterilization.18 Table 3 details the various bioactive components of milk and their biological functions.
Donor milk processing is highly relevant to clinical practice, as varying levels of bioactive compounds may contribute to disparate clinical outcomes. For example, infants who receive human milk with higher levels of HMOs have lower risks of gastroenteritis, diarrhea, and respiratory tract infections.20
Additionally, preterm infants who receive exclusive human milk feedings, including fortification, have improved growth rates11 and a lower risk for necrotizing enterocolitis.21 In addition, an exclusive human milk diet also protects against mortality and other morbidities such as sepsis and retinopathy of prematurity.22 Donor human milk is not uniform, as compositional variability has clinical implications.
A major limitation of the evidence presented is that the Meredith- Davis (2018) research is only based on 9 milk samples.18 There is tremendous need for research with larger sample sizes that are collected in a standardized format to account for the complex intrinsic milk matrix variability.
Conclusions
Clinicians need to understand the impact of various milk processing techniques on the quality and bioactive components of donor milk. Overall, there is limited literature on how/if milk compositional variability contributes to disparate neonatal outcomes. Additional studies are needed that specifically examine whether milk processing methods influence neonatal clinical outcomes. Current clinical evidence for improved neonatal outcomes is based on products using vat and/or Holder pasteurization. Because of the abundant differences noted in milk composition, it is unclear whether products undergoing retort sterilization can be expected to result in any similar outcomes.
If an institution is considering implementing a donor human milk program or changing the type of donor milk they use, it is critical for all members of the interdisciplinary team (physicians, nurses, dietitians, etc.) to understand that not all donor milk is equal. We would recommend that hospital staff read the research and understand the impact of processing methods on the milk and share the research with all members of the team so that informed decisions are being made. We also firmly believe that hospital administrators should not be making decisions about what type of “donor milk” they purchase based on cost of the product alone.
References
- Barriers to Breastfeeding: Supporting Initiation and Continuation of Breastfeeding: ACOG Committee Opinion Summary, Number 821. Obstet Gynecol. 2021;137(2):396-397. doi:10.1097/AOG.0000000000004250
- Spatz DL. Ten steps for promoting and protecting breastfeeding for vulnerable infants. J Perinat Neonatal Nurs. 2004;18(4):385-396. doi:10.1097/00005237-200410000- 0000
- Spatz DL. Beyond BFHI: The Spatz 10-step and breastfeeding resource nurse model to improve human milk
and breastfeeding outcomes. J Perinat Neonatal Nurs. 2018;32(2):164-174. doi:10.1097/JPN.0000000000000339 - Fugate K, Hernandez I, Ashmeade T, Miladinovic B, Spatz DL. Improving human milk and breastfeeding practices in the NICU. J Obstet Gynecol Neonatal Nurs. 2015;44(3):426-438; quiz E14-15. doi:10.1111/1552-6909.12563
- Kositamongkol S, Nanthakomon T, Nukaw S. A quality improvement project to improve human milk feeding rate in hospitalized neonates. J Pediatr Neonatal Indiv Med. 2019;8(1):e080111. doi:10.7363/080111
- Takako H, Mizue M, Izumi H, Chie O, Harue T, Uchida M, Spatz DL. Improving human milk and breastfeeding rates in a perinatal hospital in Japan: A quality improvement project. Breastfeed Med. 2020;15(8):538-545. doi:10.1089/bfm.2019.0298
- American Academy of Pediatrics. Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Section on Breastfeeding. Pediatrics.2017;139(1):e20163440. doi:10.1542/peds.2016.3440
- Spatz DL. Disparities in breastfeeding and use of pasteurized donor human milk. MCN Am J Matern Child Nurs.2020;45(6):374. doi:10.1097/NMC.0000000000000668
- Miller AR, Fenstermacher K, Buchko BL. Going along with it: Neonatal intensive care nurses’ views of a donor milk practice change. MCN Am J Matern Child Nurs. 2018;43(5):285-290. doi:10.1097/NMC.0000000000000454
- Abrams SA, Schanler RJ, Lee ML, Rechtman DJ. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed Med.2014;9(6):281-285. doi:10.1089/bfm.2014.0024
- Hair AB, Hawthorne KM, Chetta KE, Abrams SA. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res Notes. 2013;6:459. doi:10.1186/1756-0500-6-459
- Gopalakrishna KP, Hand TW. Influence of maternal milk on the neonatal intestinal microbiome. Nutrients. 2020;12(3):823. doi:10.3390/nu12030823
- Colaizy TT. Effects of milk banking procedures on nutritional and bioactive components of donor human milk. Semin Perinatol. 2021;45(2):151382. doi:10.1016/j.semperi.2020.151382
- Thai JD, Gregory KE. Bioactive factors in human breast milk attenuate intestinal inflammation during early life. Nutrients.2020;12(2):581. doi:10.3390/nu12020581
- Hill DR, Newburg DS. Clinical applications of bioactive milk components. Nutr Rev. 2015;73(7):463-476. doi:10.1093/nutrit/nuv009
- Resch B, ed. Neonatal bacterial infection. IntechOpen;2013.doi:10.5772/3317
- Lima HK, Wagner-Gillespie M, Perrin MT, Fogleman AD. Bacteria and bioactivity in holder pasteurized and shelf-stable human milk products. Curr Dev Nutr. 2017;1(8):e001438. doi:10.3945/cdn.117.001438
- Meredith-Dennis L, Xu G, Goonatilleke E, Lebrilla CB, Underwood MA, Smilowitz JT. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J Hum Lact. 2018;34(1):120-129. doi:10.1177/0890334417710635
- Jiang R, Lönnerdal B. Biological roles of milk osteopontin. Curr Opin Clin Nutr Metab Care. 2016;19(3):214-219. Accessed May 13, 2021. https://journals.lww.com/coclinicalnutrition/\Abstract/2016/05000/Biological_roles_of_milk_osteopontin.9.aspx#:~:text=Milk%20OPN%20affects%20 immune%20functions,similar%20to%20breast%2Dfed%20infants
- Doherty AM, Lodge CJ, Dharmage SC, Dai X, Bode L, Lowe AJ. Human milk oligosaccharides and associations with immune-mediated disease and infection in childhood: A systematic review. Front Pediatr. 2018;6:91. doi:10.3389/fped.2018.00091
- Huston RK, Markell AM, McCulley EA, Gardiner SK, Sweeney SL. Improving growth for infants ≤1250 grams receiving an exclusive human milk diet. Nutr Clin Pract. 2018;33(5):671-678. doi:10.1002/ncp.10054
- Hair AB, Peluso AM, Hawthorne KM, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet [published correction appears in Breastfeed Med. 2017;12(10):663] Breastfeed Med. 2016;11(2):70-74. doi:10.1089/bfm.2015.0134
