Press Releases
Multiple peer-reviewed studies show Prolacta’s 100% human milk-based nutritional fortifiers reduce risk of life-threatening comorbidities in premature infants, compared to cow milk-based nutrition
An Exclusive Human Milk Diet with Prolacta’s Fortifiers Reduces Risk of BPD, Late-Onset Sepsis, NEC, and ROP While Improving Neurological Development, Clinical Data Demonstrate
DUARTE, Calif., July 26, 2022 — Prolacta Bioscience®, the world’s leading hospital provider of 100% human milk-based nutritional products for critically ill, premature infants, reports that data from a growing body of peer-reviewed clinical studies show a reduced risk of several comorbidities among premature infants who received Prolacta’s 100% human milk-based fortifiers as part of Exclusive Human Milk Diet (Prolacta’s EHMD), compared to premature infants fed cow milk-based fortifiers.1,2,3,4,5,6,7,8,9,10,11,12,13
“Premature infants are susceptible to life-threatening complications that risk not only their very survival but also their long-term health and development,” said Melinda Elliott, MD, FAAP, and chief medical officer of Prolacta. “Multiple peer-reviewed studies found that Prolacta’s nutritional fortifiers, when used as part of an EHMD, play an integral role in helping reduce rates of the most common comorbidities in these fragile infants while also improving their long-term neurodevelopment.”1,2,3,4,5,6,7,8,9,10,11,12,13
Neonatal intensive care units (NICUs) worldwide are working to reduce complications in premature infants. Mounting clinical evidence shows that replacing cow milk-based fortifiers with Prolacta’s EHMD delivers the concentrated nutrition premature infants need to promote adequate growth and development,10 increases feeding tolerance,8 and reduces several of the most serious complications, including bronchopulmonary dysplasia (BPD),5,7,8 late-onset sepsis,5,6,7 necrotizing enterocolitis (NEC), 9,11,13 and retinopathy of prematurity (ROP).5,8,10
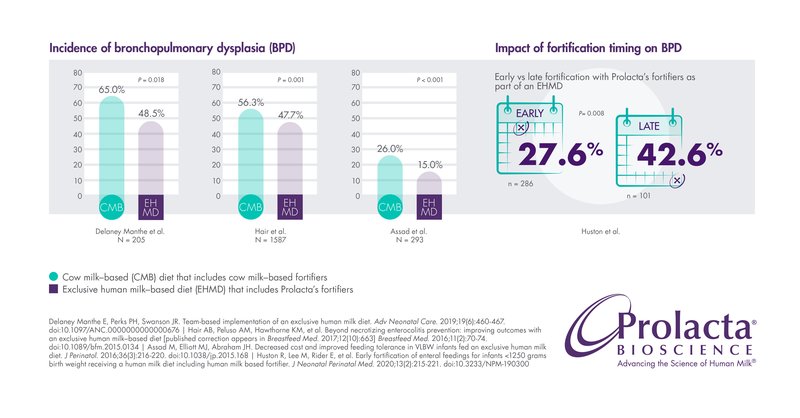
Bronchopulmonary Dysplasia (BPD)
An infant’s lungs are among the last organs to fully develop inside the womb, leaving many premature infants at high risk of developing BPD. BPD is also called chronic lung disease (CLD) of prematurity. It is one of the most serious complications of prematurity and can cause respiratory morbidity, longer hospital stays, and long-term neurodevelopmental impacts. The longer a premature infant receives supplemental oxygen or mechanical ventilation, the higher the risk of developing BPD.14
Several studies have reported significant decreases (weighted average of 9.7%) in the incidence of BPD in premature infants fed Prolacta’s EHMD compared to those fed cow milk-based nutritional products.5,7,8 Another study revealed that time is of the essence: Early fortification with Prolacta’s fortifiers as part of an EHMD led to a 15% reduction in the incidence of BPD, compared with late fortification.2
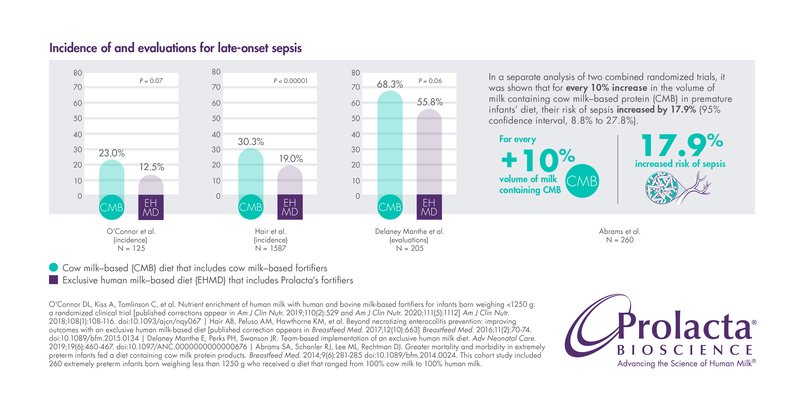
Late-Onset Sepsis
Late-onset sepsis is a dangerous systemic infection with subsequent inflammatory response and the leading cause of mortality in NICUs. It is estimated that premature infants have up to a 26% chance of developing this serious complication.5,6,7 Late-onset sepsis is associated with several potential complications, including increased ventilation use, neurodevelopmental disabilities, predisposition to other comorbidities like BPD and ROP, longer NICU stays, and higher NICU costs.2,5,7,12
Several studies show that premature infants fed Prolacta’s EHMD are less likely to develop late-onset sepsis than those fed cow milk-based fortifiers.5,6,7
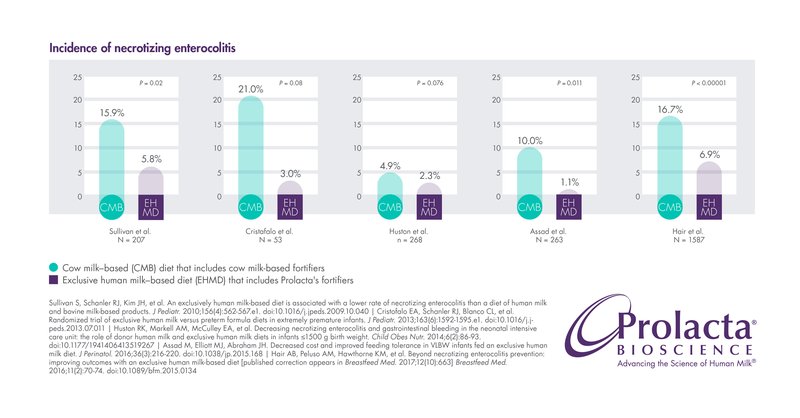
Necrotizing Enterocolitis (NEC)
NEC is a serious intestinal disease among extremely preterm infants (those born < 28 weeks gestational age) and is one of the leading causes of mortality among these very fragile, young patients.12 NEC is especially prevalent in premature infants who are fed cow milk-based fortifiers. Mortality for premature infants with NEC requiring surgical intervention can be as high as 34.9%, and 20.3% mortality in infants with medical NEC.15
Several studies have reported that premature infants who received Prolacta’s EHMD instead of cow milk-based fortifiers have a reduced incidence of medical NEC or surgical NEC. 7,8,9,11,13, 16
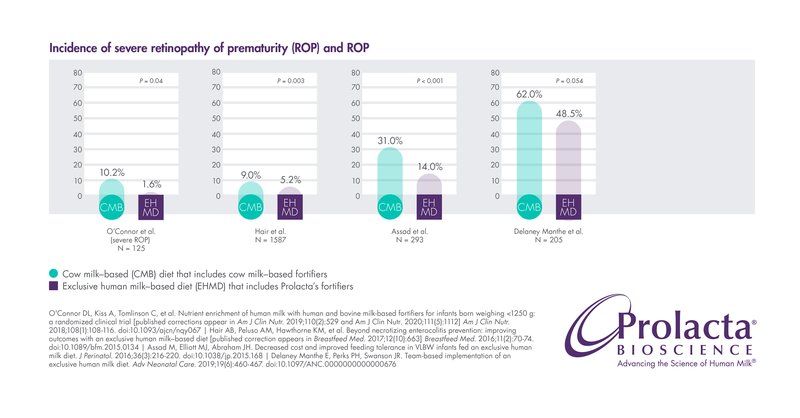
Retinopathy of Prematurity (ROP)
ROP is an eye disease primarily affecting premature infants that results in abnormal development of retinal blood vessels. Between 40% and 80% of preterm infants develop ROP, making it a leading cause of blindness and decreased vision in children worldwide with a direct impact on neurodevelopment.17,18,19,20
Multiple studies have shown that infants fed Prolacta’s EHMD have lower incidence and/or severity of ROP than those who are fed cow milk-based fortifiers.5,6,7 ,8
“The significant body of clinical evidence supporting short- and long-term health benefits with the use of an EHMD with Prolacta’s fortifiers is encouraging and positively impacts not only the infants but also their families, communities, and the health system as a whole,” said Scott Elster, president and CEO of Prolacta. “Addressing premature infant complications through nutrition in the NICU means getting these babies home sooner and healthier, and ultimately reducing hospital costs.”8,12 ,21
In addition to reducing the risk of life-threatening comorbidities, Prolacta’s EHMD has been clinically shown to:
- Lower mortality and morbidity7,8,9,11
- Achieve adequate growth2,4,9,10,22
- Shorten stays in the NICU8
- Reduce hospital costs8,12 ,21
- Improve long-term outcomes such as neurological development1,3
- Reduce feeding intolerance8
About Human Milk-Based Products
The major difference between cow milk-based and human milk-based products is the composition — notably, the bioactive components that are unique to human milk. These include immunoglobulins, lactoferrin, milk fat globule membrane, and the wide spectrum of prebiotics known as human milk oligosaccharides (HMOs), which are not easily manufactured and thus are greatly decreased or missing from cow milk-based nutritional products.23 Bioactivity is thought to support infants’ immunity, development, growth, and long-term health.24
Prolacta’s 100% human milk-based nutritional products have the highest bioactivity in the human milk industry.25 Prolacta’s nutritional products are vat pasteurized using profiles defined by the U.S. Food and Drug Administration (FDA) to ensure pathogen inactivation and the highest level of safety while retaining as much of the natural bioactivity of the milk as possible.25 Prolacta's vat pasteurized products retain higher bioactivity than products processed using other methods, including retort sterilization and ultra-high-temperature (UHT) processing.26,27
About Prolacta Bioscience
Prolacta Bioscience® Inc. is a privately held, global life sciences company dedicated to Advancing the Science of Human Milk® to improve the health of critically ill, premature infants. Prolacta's 100% human milk-based nutritional products have been evaluated in more than 20 clinical studies published in peer-reviewed journals. More than 80,000 premature infants have benefited from Prolacta’s nutritional products worldwide to date.* Established in 1999, Prolacta is the world’s leading provider of human milk-based nutritional products for hospital use and is also exploring the therapeutic potential of human milk across a wide spectrum of diseases. Prolacta maintains the industry’s strictest quality and safety standards for screening, testing, and processing human donor milk. Operating the world’s first pharmaceutical-grade human milk processing facilities, Prolacta uses vat pasteurization and a patented, U.S. Food and Drug Administration-reviewed manufacturing process to ensure pathogen inactivation while protecting the nutritional composition and bioactivity of its human milk-based products. Prolacta is a global company with headquarters in Duarte, California, and can be found on Twitter, Instagram, Facebook, and LinkedIn.
*Estimated number of premature infants fed Prolacta’s products from January 2007 to December 2021; data on file.
# # #
Media Contact:
Loren Kosmont
Lkosmont@prolacta.com
310-721-9444
References
- Bergner EM, Shypailo R, Visuthranukul C, et al. Growth, body composition, and neurodevelopmental outcomes at 2 years among preterm infants fed an exclusive human milk diet in the neonatal intensive care unit: a pilot study. Breastfeed Med. 2020. 15(5):304-311. doi:10.1089/bfm.2019.0210
- Huston R, Lee M, Rider E, et al. Early fortification of enteral feedings for infants <1250 grams birth weight receiving a human milk diet including human milk-based fortifier. J Neonatal Perinatal Med. 2020;13(2):215-221. doi:10.3233/NPM-190300
- Rahman A, Kase J, Murray Y, et al. Neurodevelopmental outcome of extremely low birth weight infants fed an exclusive human milk diet is not affected by growth velocity. Breastfeed Med. 2020;15(6):362-369. doi:10.1089/bfm.2019.0214
- Lucas A, Boscardin J, Abrams SA. Preterm infants fed cow's milk-derived fortifier had adverse outcomes despite a base diet of only mother's own milk. Breastfeed Med. 2020;15(5):297-303. doi:10.1089/bfm.2019.013
- Delaney Manthe E, Perks PH, Swanson JR. Team-based implementation of an exclusive human milk diet. Adv Neonatal Care. 2019;19(6):460-467. doi:10.1097/ANC.0000000000000676
- O'Connor DL, Kiss A, Tomlinson C, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial [published corrections appear in Am J Clin Nutr. 2019;110(2):529 and Am J Clin Nutr. 2020;111(5):1112] Am J Clin Nutr. 2018;108(1):108-116. doi:10.1093/ajcn/nqy067
- Hair AB, Peluso AM, Hawthorne KM, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet [published correction appears in Breastfeed Med. 2017;12(10):663] Breastfeed Med. 2016;11(2):70-74. doi:10.1089/bfm.2015.0134
- Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36(3):216-220. doi:10.1038/jp.2015.168
- Abrams SA, et al. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed Med. June 2014. 9(6): 281-0285. doi:10.1089/bfm.2014.0024. This cohort study included 260 extremely preterm infants born weighing less than 1,250 g who received a diet that ranged from 100% cow milk to 100% human milk.
- Hair AB, Hawthorne KM, Chetta KE, et al. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res Notes 2013;6:459. doi:10.1186/1756-0500-6-459.
- Cristofalo EA, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. December 2013. 163(6):1592-1595. doi: 10.1016/j.jpeds.2013.07.011.
- Ganapathy V, et al. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med. February 2012. 7(1):29-37. doi: 10.1089/bfm.2011.0002. This cost-effectiveness analysis of 2,560 extremely premature infants less than 28 weeks gestational age in 257 hospitals nationwide compared the impact of an exclusive human milk diet composed of mother’s milk fortified with a human milk-based fortifier versus mother’s milk fortified with cow milk-based fortifier.
- Sullivan S, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. April 2010. 156(4):562-567. doi: 10.1016/j.jpeds.2009.10.040.
- Learn about bronchopulmonary dysplasia. American Lung Association. Accessed March 6, 2020. https://www.lung.org/lung-health-diseases/lung-disease-lookup/bronchopulmonary-dysplasia/learn-about-bpd.
- Han SM, Hong CR, Knell J, et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: A multicenter cohort analysis. J Pediatr Surg. 2020;55(6):998-1001. doi:10.1016/j.jpedsurg.2020.02.046
- Huston RK, et al. Decreasing necrotizing enterocolitis and gastrointestinal bleeding in the neonatal intensive care unit: the role of donor human milk and exclusive human milk diets in infants <1,500 g birth weight. Infant Child Adolesc Nutr. 10 January 2014. 13:127. doi: 10.1177/1941406413519267.
- Dutta S, Raghuveer T, Vinekar A, Dogra MR. Can we stop the current epidemic of blindness from retinopathy of prematurity? Indian Pediatr. 2016;53(Suppl 2):S80-S84.
- Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J AAPOS. 2012;16(6):501-507. doi:10.1016/j.jaapos.2012.09.004
- Raghuveer TS, Zackula R. Strategies to prevent severe retinopathy of prematurity: a 2020 update and meta-analysis. NeoReviews. 2020;21(4):e249-e263. doi:10.1542/neo.21-4-e249
- Molloy CS, Anderson PJ, Anderson VA, Doyle LW. The long-term outcome of extremely preterm (<28 weeks’ gestational age) infants with and without severe retinopathy of prematurity. J Neuropsychol. 2016;10(2):276-294. doi:10.1111/jnp.12069
- Iskersky V. Use of an exclusive human milk diet in preterm infants to lower healthcare costs – short-term and long-term perspectives. Neonatal Intensive Care. 2020:37-39.
- Huston RK, Markell AM, McCulley EA, Gardiner SK, Sweeney SL. improving growth for infants ≤1250 grams receiving an exclusive human milk diet. Nutr Clin Pract. 2018;33(5):671-678. doi:10.1002/ncp.10054.
- Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013 Feb;60(1):49-74. doi: 10.1016/j.pcl.2012.10.002. PMID: 23178060; PMCID: PMC3586783.
- Gila-Diaz A, Arribas SM, Algara A, Martín-Cabrejas MA, López de Pablo ÁL, Sáenz de Pipaón M, Ramiro-Cortijo D. A review of bioactive factors in human breastmilk: a focus on prematurity. Nutrients. 2019;11(6):1307. doi:10.3390/nu11061307
- Internal data.
- Lima HK, Wagner-Gillespie M, Perrin MT, Fogleman AD. Bacteria and bioactivity in Holder pasteurized and shelf-stable human milk products. Curr Dev Nutr. 2017;1(8):e001438. doi:10.3945/cdn.117.001438
- Meredith-Dennis L, Xu G, Goonatilleke E, Lebrilla CB, Underwood MA, Smilowitz JT. Composition and variation of macronutrients, immune proteins, and human milk oligosaccharides in human milk from nonprofit and commercial milk banks. J Hum Lact. 2018;34(1):120-129. doi:10.1177/0890334417710635
