Product Information
Donor human milk brochure
Human milk makes all the difference for premature infants. Prolacta Bioscience offers guaranteed supply* of quality donor human milk to supplement mother's own milk.
Donor Human Milk
Pasteurized. Standardized. Quality Assured.
The benefits of breastfeeding are well established and highly recommended by healthcare professionals and the U.S. Department of Health and Human Services (HHS).1 Mother’s own milk (MOM) and donor human milk (when MOM is not available) are rapidly becoming the standard of care for feeding all premature infants. Your neonatal intensive care unit (NICU) can depend on Prolacta for the safest, highest-quality donor human milk—with no supply shortages.
- “Breastfeeding and human milk are the normative standards for infant feeding and nutrition.”
- “The potent benefits of human milk are such that all preterm infants should receive human milk.”
- “If mother’s own milk is unavailable … pasteurized donor milk should be used.”
— American Academy of Pediatrics (AAP) Policy on Breastfeeding and the Use of Human Milk2
Lower pricing options with committed purchase agreements.
*With a committed purchase agreement
Prolacta is the industry leader in providing standardized human milk.
Standardized nutrition
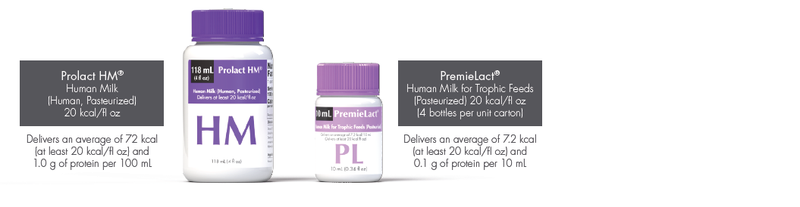
- The first donor human milk formulated to deliver an average of 72 kcal (at least 20 kcal/fl oz) and 1.0 g of protein per 100 mL3
- Labeled in accordance with U.S. Food and Drug Administration (U.S. FDA) food labeling requirements
- Two-year shelf life supported by real-time stability studies3
- Should be administered within 48 hours once the thawing process begins3
Industry-leading quality and safety
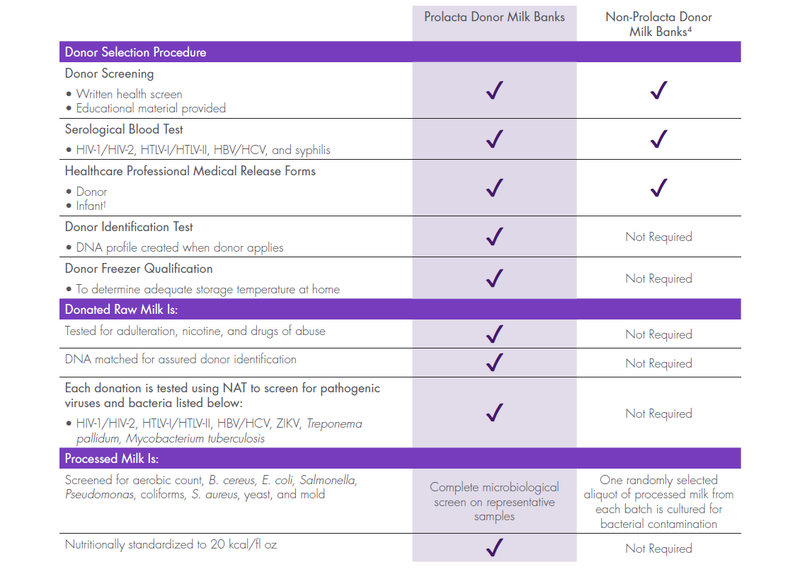
- Donor human milk verified by deoxyribonucleic acid (DNA) matching to the donor
- Each donation tested for common drugs of abuse, nicotine, and adulteration
- Each donation tested using nucleic acid amplification testing (NAT) for pathogenic viruses and bacteria listed below:
— Human immunodeficiency virus type 1 and type 2 (HIV-1/HIV-2)
— Human T-lymphotropic virus type I and type II (HTLV-I/HTLV-II)
— Hepatitis virus type B and type C (HBV/HCV)
— Zika virus (ZIKV)
— Treponema pallidum
— Mycobacterium tuberculosis
- Each donation processed in a pharmaceutical-grade manufacturing facility
Guaranteed supply*
- Prolacta offers a guaranteed supply of donor human milk based on your NICU’s usage forecast—that means no more donor human milk shortages
*With a committed purchase agreement
Available in two sizes for flexibility in feeding your premature infants:
Prolacta is committed to raising the bar on quality and safety.
“ There are significant risks involved in the collection, processing, and distribution of donor milk–based products. The behaviors of the donors, biochemical and genetic screening, and milk processing are critical to mitigation of these recognized risks. Testing at this level of rigor appears to be justified.”
—Bloom Report on Safety of Donor Milk5
References:
†Exception if baby is not in their care, such as the baby has died or been given up for adoption
1 U.S. Food and Drug Administration. Use of donor human milk. https://www.fda.gov/science-research/pediatrics/use-donor-human-milk. Updated March 22, 2018. Accessed April 6, 2020
2 American Academy of Pediatrics. Breastfeeding and the use of human milk. Section on Breastfeeding. Pediatrics. 2012;129(3):e827-e841. doi:10.1542/peds.2011-3552
3 Data on file.
4 Guidelines for the establishment and operation of a donor human milk bank. 10th ed. Human Milk Banking Association of North America. 2018:15-16,43-44,47.
5 Bloom BT. Safety of donor milk: a brief report. J Perinatol. 2016;36(5):392-393. doi:10.1038/jp.2015.207
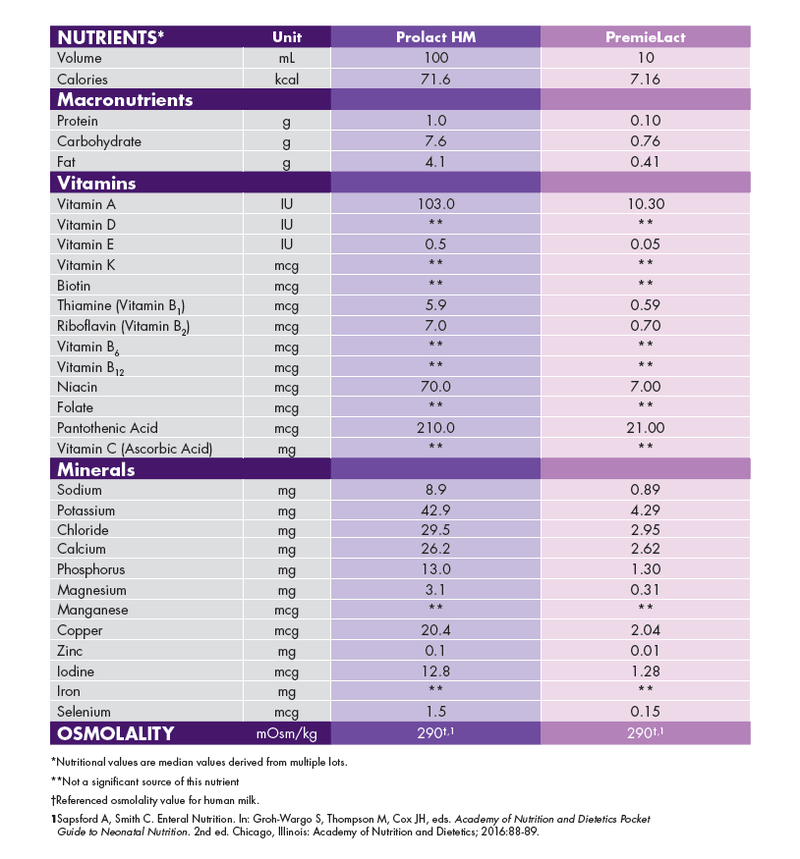
Prolact HM donor human milk (118 mL) and PremieLact donor human milk (10 mL) Nutrition Information.