Fact Sheet
Health complications have cost brochure
Addressing Nutritional Risks for Premature Infants:
Health Complications Have Costs
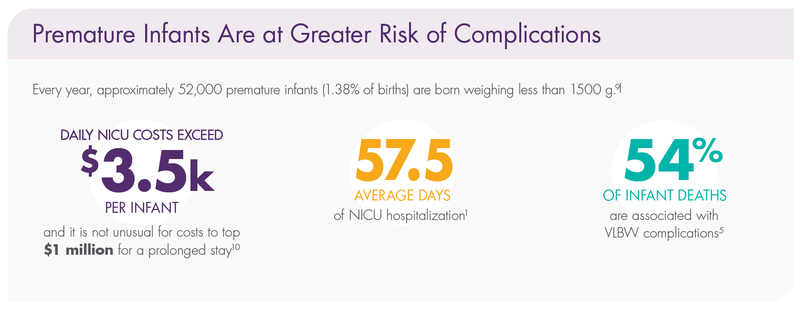
Very low birth weight (VLBW) premature infants spend an average of 57.5 days1 in the neonatal intensive care unit (NICU). The earlier a baby is born, the more severe the nutritional risks and health problems can be.
An exclusive human milk diet (EHMD) can increase survival2 and reduce the overall cost of care for premature infants weighing ≤1250 g at birth.3 With Prolacta’s products as part of an EHMD (Prolacta’s EHMD),† you can decrease costly complications—such as necrotizing enterocolitis—associated with cow milk–based products.4
Costly Health Complications
VLBW premature infants are at risk for morbidities, such as:
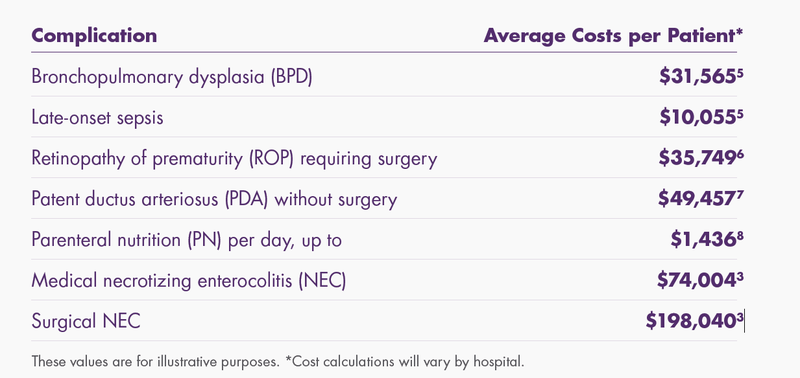
The incremental costs of these morbidities and interventions can substantially increase the cost of a NICU hospitalization.
Premature Infants Are at Greater Risk of Complications
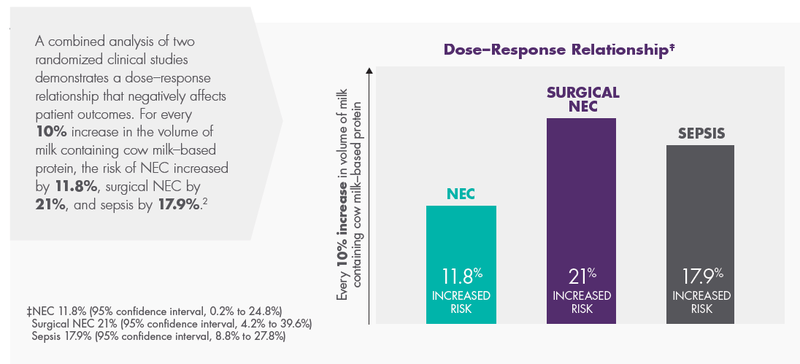
A Cow Milk–Based Diet (CMD) Can Increase Medical Complications and Costs
Preterm Infants Have Greater Nutritional Needs
Premature infants have significant nutritional requirements including increased calories, protein, vitamins, calcium, and other minerals. Nutrition is vital to their survival, growth, and development. During the last trimester, infants receive vast amounts of essential nutrition through the umbilical cord. Premature infants miss this crucial nutrition, and their dietary needs are greater than what breastmilk alone can supply.
For premature infants weighing less than 1500 g at birth, the American Academy of Pediatrics (AAP) recommends fortifying human milk (mother’s own milk [MOM] or pasteurized donor human milk) with protein, minerals, and vitamins to ensure optimal nutrition intake.11
When Addressing the Nutritional Risks for Premature Infants, Only Prolacta Offers a Full Line of Clinically Proven, Human Milk–Based Nutrition
Decrease Costly Health Complications and Support Healthy, Long-Term Growth With Prolacta’s EHMD
“Human Milk Fortifier” (HMF) is the generic name of a product that aims to increase the nutritional and caloric density of MOM or pasteurized donor human milk to meet the dietary needs of premature babies in the NICU. Some people assume that “Human Milk
Fortifier” means the product is made from human milk, which is not the case. There are two categories of HMF available for use in NICUs in the United States: one derived from cow’s milk and the other from pasteurized donor human milk. HMF does not reflect which category of fortifier is being used.12
Prolacta’s EHMD is achieved when 100% of protein, fat, and carbohydrate are derived from MOM and/or pasteurized donor milk, along with Prolacta’s human milk and human milk–based fortifiers, caloric fortifiers, and premature infant formulas.
Prolacta’s EHMD for babies weighing ≤1250 g at birth supports adequate growth,13 can increase survival,2 and can decrease costly complications associated with the intake of cow milk–based products.3,4
Improved survival rates of premature infants have shifted the focus of neonatal care to improving postnatal growth and nutrition and aiming to achieve growth rates that optimize later health outcomes.14,15,16
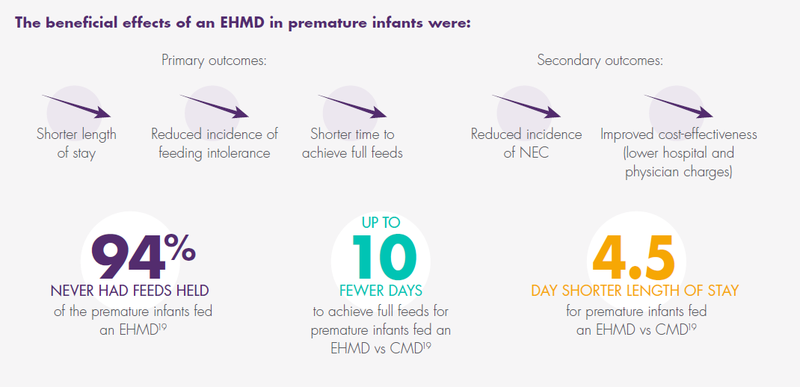
An EHMD feeding protocol for premature infants weighing <1500 g with early and rapid advancement of fortification with Prolacta’s products is associated with:
Prolacta’s Products Can Help Reduce the Most Severe Complications of Prematurity
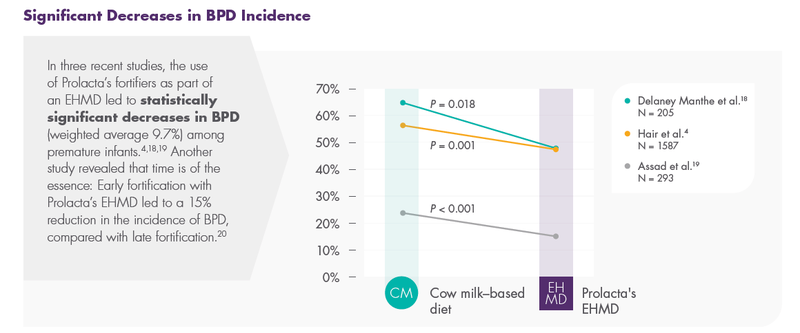
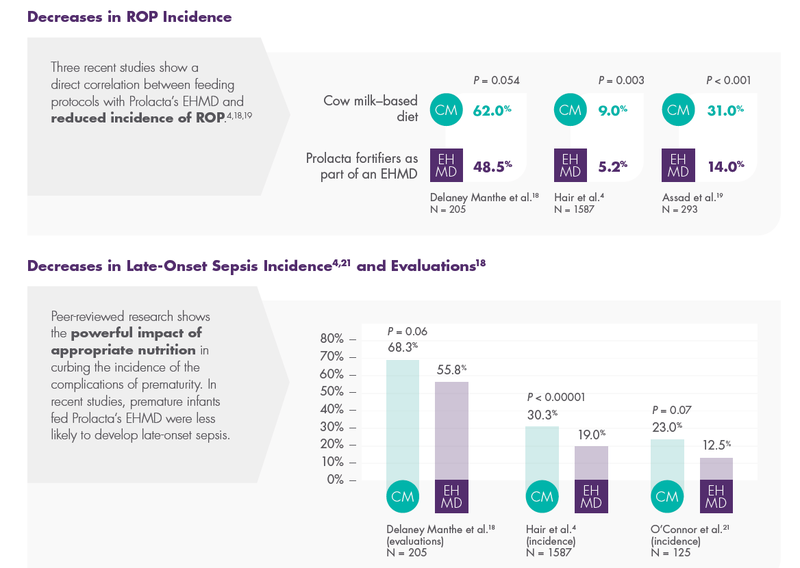
Prolacta’s EHMD, devoid of cow milk–based products, has been shown to lower mortality and comorbidities in premature infants ≤1250 g without compromising growth.2,17
In extremely premature infants given an EHMD vs preterm formula, there was a significant difference in median parenteral nutrition days: 27 vs 36 days with Prolact+ H2MF® human milk fortifiers (human, pasteurized) vs bovine milk–based preterm formula, respectively (P = 0.04).22§
Prolacta’s EHMD Can Reduce Hospital Costs19
The 2016 Assad et al single-center retrospective study assessed the benefits and costs of Prolacta’s EHMD in preterm (≤28 weeks) and/or VLBW (≤1500 g) infants vs either a combination of MOM and cow milk–based fortifier; a mixed combination diet of MOM, cow milk–based fortifier, and formula; or a formula-only diet.
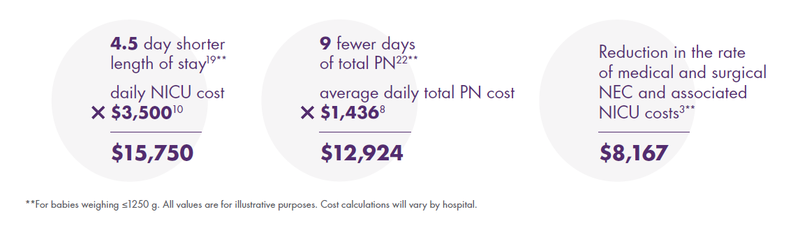
Potential Average Cost Savings per Premature Infant by Addressing Nutritional Risks With Prolacta’s EHMD
Long-Term Cost-Effectiveness of Prolacta’s EHMD
Morbidities associated with prematurity predispose VLBW infants to long-term complications and increase risk of neurodevelopmental and neurocognitive problems that can add to healthcare system and societal costs.5
From a financial perspective, Prolacta’s EHMD for preterm infants in the NICU has the potential for cost savings that will span their lifetime and result in healthcare system cost savings over the long term.3,23

1 Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants.
J Pediatr. 2013;162(2):243-249. doi:10.1016/j.jpeds.2012.07.013
2 Abrams SA, Schanler RJ, Lee ML, Rechtman DJ. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed Med. 2014;9(6):281- 285. doi:10.1089/bfm.2014.0024
3 Ganapathy V, Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med. 2012;7(1):29-37. doi:10.1089/bfm.2011.0002
4 Hair AB, Peluso AM, Hawthorne KM, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet [published correction appears in Breastfeed Med. 2017;12 (10):663]. Breastfeed Med. 2016;11(2):70-74. doi:10.1089/bfm.2015.0134
5 Johnson TJ, Patel AL, Bigger HR, Engstrom JL, Meier PP. Economic benefits and costs of human milk feedings: a strategy to reduce the risk of prematurity-related morbidities in very-low-birth-weight infants. Adv Nutr. 2014;5(2):207-212. doi:10.3945/an.113.004788
6 Black L, Hulsey T, Lee K, Parks DC, Ebeling MD. Incremental hospital costs associated with comorbidities of prematurity. Manag Care. 2015;24(12):54-60.
7 Turck CJ, Marsh W, Stevenson JG, York JM, Miller H, Patel S. Pharmacoeconomics of surgical interventions vs. cyclooxygenase inhibitors for the treatment of patent ductus arteriosus. J Pediatr Pharmacol Ther. 2007;12(3):183-193. doi:10.5863/1551-6776-12.3.183
8 Edwards TM, Spatz DL. Making the case for using donor human milk in vulnerable infants. Adv Neonatal Care. 2012;12(5):273-280. doi:10.1097/ANC.0b013e31825eb094
9 Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Division of Vital Statistics. Births: Final Data for 2018. Natl Vital Stat Rep. 2019;68(13):1-47.
10 Muraskas J, Parsi K. The cost of saving the tiniest lives: NICUs versus prevention. Virtual Mentor. 2008;10(10):655-658. doi:10.1001/virtualmentor.2008.10.10.pfor1-0810
11 American Academy of Pediatrics. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi:10.1542/peds.2011-3552
12 Canvasser J, Hair AB, Kim JH, Taylor SN. Parent and prescriber perspectives on the imprecise label of “human milk fortifier” in the NICU. Nutrients. 2020;12(3):720. doi:10.3390/nu12030720
13 Hair AB, Hawthorne KM, Chetta KE, Abrams SA. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res Notes. 2013;6:459. doi:10.1186/1756-0500-6-459
14 Rochow N, Raja P, Liu K, et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr Res. 2016;79(6):870-879. doi:10.1038/pr.2016.15
15 Visuthranukul C, Abrams SA, Hawthorne KM, Hagan JL, Hair AB. Premature small for gestational age infants fed an exclusive human milk-based diet achieve catch-up growth without metabolic consequences at 2 years of age. Arch Dis Child Fetal Neonatal Ed. 2019;104(3):F242-F247. doi:10.1136/archdischild-2017-314547
16 Bergner EM, Shypailo R, Visuthranukul C, et al. Growth, body composition, and neurodevelopmental outcomes at 2 years among preterm infants fed an exclusive human milk diet in the neonatal intensive care unit: a pilot study. Breastfeed Med. 2020;15(5):304-311. doi:10.1089/bfm.2019.0210
17 Lucas A, Boscardin J, Abrams SA. Preterm infants fed cow’s milk-derived fortifier had adverse outcomes despite a base diet of only mother’s own milk. Breastfeed Med. 2020;15(5):297-303. doi:10.1089/bfm.2019.0133
18 Delaney Manthe E, Perks PH, Swanson JR. Team-based implementation of an exclusive human milk diet. Adv Neonatal Care. 2019;19(6):460-467. doi:10.1097/ANC.0000000000000676
19 Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36(3):216-220. doi:10.1038/jp.2015.168
20 Huston R, Lee M, Rider E, et al. Early fortification of enteral feedings for infants <1250 grams birth weight receiving a human milk diet including human milk based fortifier. J Neonatal Perinatal Med. 2020;13(2):215-221. doi:10.3233/NPM-190300
21 O’Connor DL, Kiss A, Tomlinson C, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial [published corrections appear in Am J Clin Nutr. 2019;110(2):529 and Am J Clin Nutr. 2020;111(5):1112]. Am J Clin Nutr. 2018;108(1):108-116. doi:10.1093/ajcn/nqy067
22 Cristofalo EA, Schanler RJ, Blanco CL, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592-1595. e1. doi:10.1016/j.jpeds.2013.07.011
23 Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562-567.e1. doi:10.1016/j.jpeds.2009.10.040
