Fact Sheet
Fact sheet: Retinopathy of prematurity clinical information
Retinopathy of Prematurity:
Reducing Risk, Improving Outcomes With Prolacta’s Exclusive Human Milk Diet
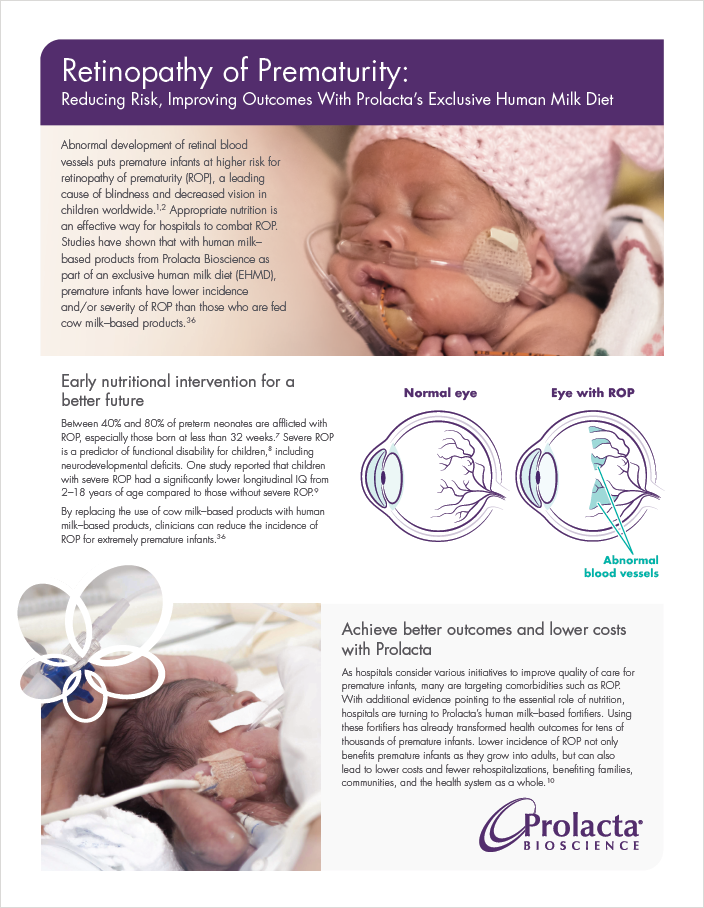
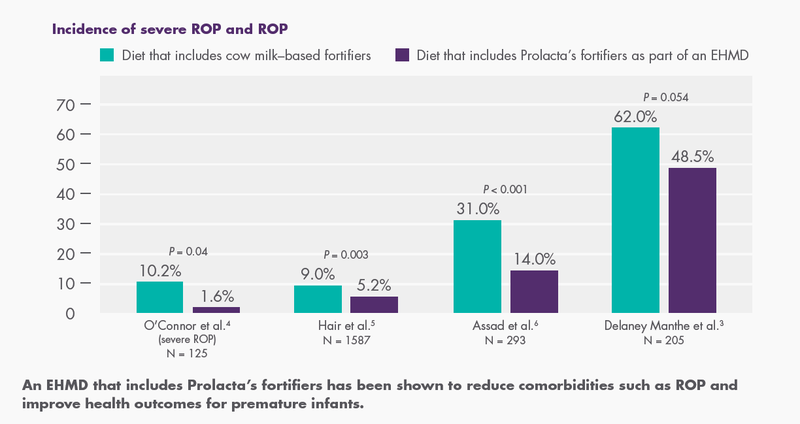
Abnormal development of retinal blood vessels puts premature infants at higher risk for retinopathy of prematurity (ROP), a leading cause of blindness and decreased vision in children worldwide.1,2 Appropriate nutrition is an effective way for hospitals to combat ROP. Studies have shown that with human milk–based products from Prolacta Bioscience as part of an exclusive human milk diet (EHMD), premature infants have lower incidence and/or severity of ROP than those who are fed cow milk–based products.3,4,5,6
Early nutritional intervention for a better future
Between 40% and 80% of preterm neonates are afflicted with ROP, especially those born at less than 32 weeks.7 Severe ROP is a predictor of functional disability for children,8 including neurodevelopmental deficits. One study reported that children with severe ROP had a significantly lower longitudinal IQ from 2–18 years of age compared to those without severe ROP.9
By replacing the use of cow milk–based products with human milk–based products, clinicians can reduce the incidence of ROP for extremely premature infants.3,4,5,6
Achieve better outcomes and lower costs with Prolacta
As hospitals consider various initiatives to improve quality of care for premature infants, many are targeting comorbidities such as ROP. With additional evidence pointing to the essential role of nutrition, hospitals are turning to Prolacta’s human milk–based fortifiers. Using these fortifiers has already transformed health outcomes for tens of thousands of premature infants. Lower incidence of ROP not only benefits premature infants as they grow into adults, but can also lead to lower costs and fewer rehospitalizations, benefiting families, communities, and the health system as a whole.10
What the research shows
In four studies, including a randomized controlled trial, Prolacta’s EHMD has been shown to decrease the incidence and/or severity of ROP in preterm infants weighing <1250 g, compared with cow milk–based fortifiers.3,4,5,6
References:
- Dutta S, Raghuveer T, Vinekar A, Dogra MR. Can we stop the current epidemic of blindness from retinopathy of prematurity? Indian Pediatr. 2016;53(suppl 2):S80-S84.
- Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J AAPOS. 2012;16(6):501-507. doi:10.1016/j.jaapos.2012.09.004
- Delaney Manthe E, Perks PH, Swanson JR. Team-based implementation of an exclusive human milk diet. Adv Neonatal Care. 2019;19(6):460-467. doi:10.1097/ANC.0000000000000676
- O’Connor DL, Kiss A, Tomlinson C, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108(1):108-116. doi:10.1093/ajcn/nqy067. Published corrections appear in Am J Clin Nutr. 2019;110(2):529. doi:10.1093/ajcn/nqz091 and Am J Clin Nutr. 2020;111(5):1112. doi:10.1093/ajcn/nqaa042
- Hair AB, Peluso AM, Hawthorne KM, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed Med. 2016;11(2):70-74. doi:10.1089/bfm.2015.0134. Published correction appears in Breastfeed Med. 2017;12(10):663. doi:10.1089/ bfm.2015.0134.correx
- Assad M, Elliott MJ, Abraham JH. Decreased cost and improved feeding tolerance in VLBW infants fed an exclusive human milk diet. J Perinatol. 2016;36(3):216-220. doi:10.1038/jp.2015.168
- Manzoni P, Stolfi I, Pedicino R, et al. Human milk feeding prevents retinopathy of prematurity (ROP) in preterm VLBW neonates. Early Hum Dev. 2013;89(Suppl 1):S64-S68. doi:10.1016/S0378-3782(13)70019-7
- Raghuveer TS, Zackula R. Strategies to prevent severe retinopathy of prematurity: a 2020 update and meta-analysis. NeoReviews. 2020;21(4):e249-e263. doi:10.1542/neo.21-4-e249
- Molloy CS, Anderson PJ, Anderson VA, Doyle LW. The long-term outcome of extremely preterm (<28 weeks’ gestational age) infants with and without severe retinopathy of prematurity. J Neuropsychol. 2016;10(2):276-294. doi:10.1111/jnp.12069
- Johnson TJ, Patel AL, Bigger HR, Engstrom JL, Meier PP. Economic benefits and costs of human milk feedings: a strategy to reduce the risk of prematurity-related morbidities in very-low-birth-weight infants. Adv Nutr. 2014;5(2):207-212. doi:10.3945/an.113.004788
- Estimated number of premature infants fed Prolacta’s products from January 2007 to August 2020; data on file.
About Prolacta Bioscience
Established in 1999, we created the first and only neonatal nutritional fortifiers made from 100% human milk, rather than cow milk. Based in California with employees throughout the world, we’re a privately held life sciences company that’s touched the lives of more than 63,000 premature infants globally.11 We operate the first and only pharmaceutical-grade manufacturing facilities for testing and processing donor milk. We exceed food-product industry requirements by following stringent quality and safety standards based on those for the human blood and plasma industry.
