Clinically shown, human milk–based nutrition
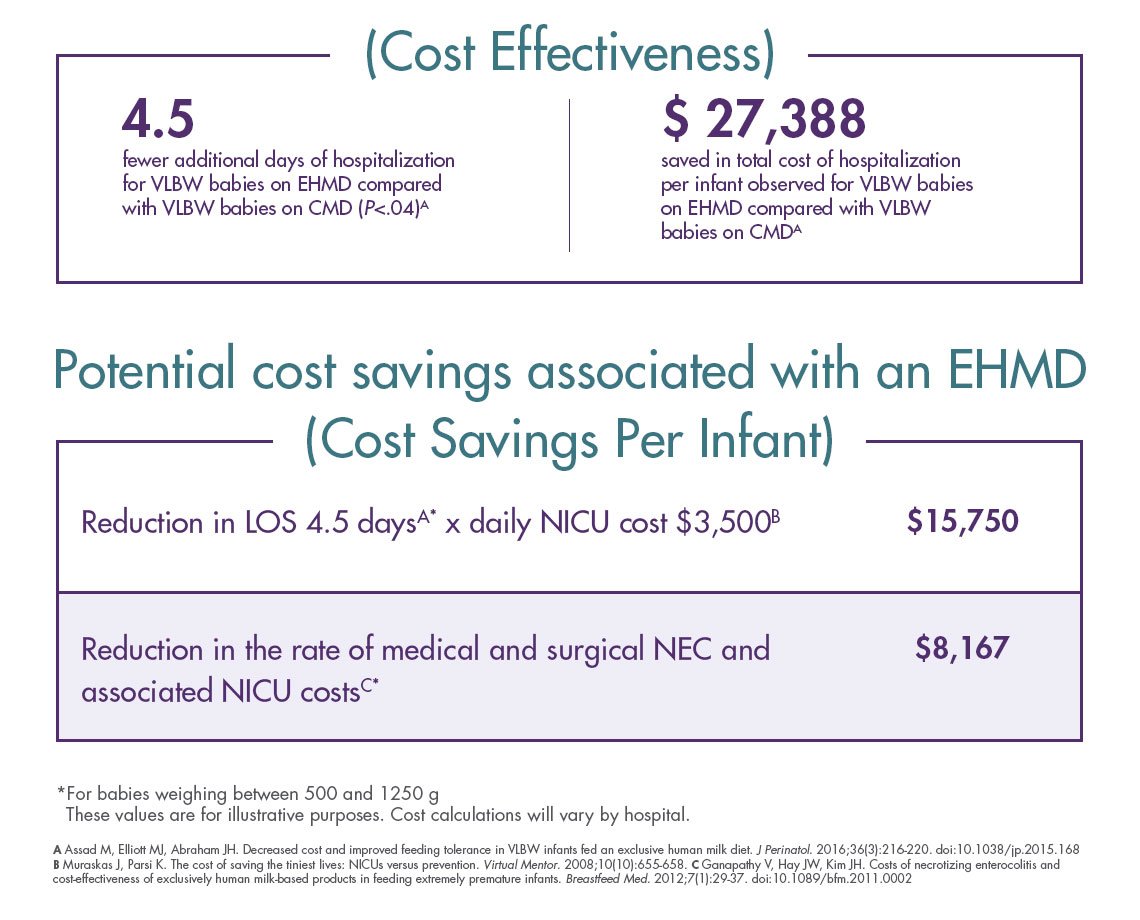
When used as part of an exclusive human milk diet (EHMD) for preterm infants as compared to cow milk–based products, Prolacta’s products have been shown in clinical studies to :
Premature infant nutrition
Fortifier
Supplementing mother’s own milk (MOM) with the first commercially available human milk–based fortifier made from 100% human donor milk instead of cow milk
Caloric fortifier
When premature infants need additional calories to support their growth, this human milk–based caloric fortifier delivers
Ready-to-feed
This ready-to-feed, human milk–based product offers NICUs an alternative to cow milk-based formula when MOM is not available
Human donor milk
When an adequate supply of MOM is not available, donor milk is rapidly becoming the standard of care for feeding premature infants
Term infant nutrition
Term babies with gastroschisis have special nutritional needs considering their delicate gastrointestinal system. When recovering from surgery, these term babies need human milk and may need human-milk based fortifiers for additional nutrition to help recover from surgery.



Adequately nourishing infants with mother’s own milk (MOM) fortified with human milk–based products
Since 2012, the American Academy of Pediatrics (AAP) has recommended human milk for preterm infants, whether MOM or pasteurized donor human milk if MOM is unavailable.14 For those infants born weighing less than 1500 g, the AAP recommends fortifying MOM or donor human milk with protein and minerals to ensure optimal nutrient intake. 15
In 2025, the US Food and Drug Administration approved the first human milk-based fortifier for term infants recovering from surgery for gastroschisis.
Our products help hospitals meet the nutritional needs of their medically fragile patients.

Made with lab-tested donor milk in U.S. FDA-regulated manufacturing facilities
Hospitals choose Prolacta’s 100% human milk–based nutritional products for the assurance that they are receiving safe, standardized donor milk formulations processed in a pharmaceutical-grade facility under the strictest quality and safety guidelines to protect the health and well-being of their most fragile patients1.